Wave-Particle Duality
March 11, 2024
Television was generally
monochrome (black and white) until the
mid-1960s. While
color television had been demonstrated using various
technologies decades earlier, there was a desire for a method that would be
backwards-compatible with existing
television receivers; that is, color
transmissions would appear as black and white on existing receivers. The end result was a system that represented color as a small additional
signal detected with
reference to a
local oscillator stabilized by a
signal pulse between
image frames.
There was a memorable series of black and white
television commercials in the 1960s that featured
teenage twins debating about whether
Certs was a
breath mint or a
candy mint.[1] This question was
resolved by an
announcer declaring Certs to be
both a candy mint
and a breath mint; viz.,
Twin No. 1 - "Certs is a candy mint!"
Twin No. 2 - "Certs is a breath mint!"
Announcer - "Stop! You're both right! New Certs is two mints in one!"

Certs twins.
In 1999, the United States Customs Service decided that Certs was a candy, and it placed it into the highly taxed tariff class 2106.90.99.
Warner-Lambert, owner of Certs at that time, won an appeal to the Court of Appeals for the Federal Circuit that Certs should be placed in tariff class 3306.90.00, "Preparations for oral or dental hygiene;" so, Certs is legally a breath mint.[2]
(Still image from a YouTube video. Click for larger image.)
A similar
debate about the
nature of
electrons happened decades earlier. This debate was whether electrons, and other
matter, were
particles or
waves. That debate had the same resolution - They're both particles and waves depending on how you
measure them. This principle was first
hypothesized by
French physicist,
Louis de Broglie (1892-1987), formally, Louis-Victor-Pierre-Raymond, Seventh duc de Broglie.[3] De Broglie knew, from the work of
Hermann Minkowski (1864-1909) and
Max Abraham (1875-1922) that the
vacuum wavelength λ of a
photon is the
Planck constant h divided by its
momentum p; that is,
λ = h/p
You can see how this is possible by
equating the photon
energy from the
Einstein equation for
mass-energy equivalence with the energy in the
Planck relation; viz.,
E= mc2 = hν
λ = c/ν
λ = h/mc
The term
mc is the photon momentum
p. Generalizing this to particles other than phonons replaces the
speed of light c in the momentum relation with the
velocity v.
λ= h/p = h/mv

French physicist, Louis de Broglie (1892-1987) (formally, Louis-Victor-Pierre-Raymond, Seventh duc de Broglie) in 1929.
De Broglie might have been lost to science, since he received his first degree in history. However, he became interested in mathematics and physics and was awarded a Ph.D. in 1924 for the thesis, "Recherches sur la théorie des quanta" (Research on the Theory of the Quanta).[3] This thesis introduced his concept that matter has wave properties.
(Modified Wikimedia Commons image.)
The wave nature of electrons was just an
hypothesis until proven by a definitive
experiment on electron diffraction in 1927 by
Clinton Davisson (1881-1958) and
Lester Germer (1896-1971) at
Bell Labs. Davisson and Germer
scattered electrons from the
surface of a
crystal of
nickel to display a
diffraction pattern in an
analogy of
X-ray diffraction by matter instead of
electromagnetic waves. De Broglie was awarded the 1929
Nobel Prize in Physics "
for his discovery of the wave nature of electrons."[4] Davisson shared the 1937 Nobel Prize in Physics for the discovery of
electron diffraction, but Germer was notably slighted.
The
quantum nature of matter has been experimentally proven for electrons (
mass = 9.11 x 10
-28 grams) and particles up to 10
-20 grams.[5-6] Physicist generally believe that larger masses will demonstrate quantum effects. However, such effects are very small, and they are harder to determine experimentally, since a larger mass has many interactions with the
environment that will
collapse its quantum state.[6] There is no apparent mass limit to quantum mechanics, but, as yet, no definitive experiment to confirm the quantumness of an arbitrarily large mass.[5-6]
One promising experiment has been proposed in a recent
article in
Physical Review letters by a team of physicists from
University College London (London, United Kingdom), the
Bose Institute (Kolkata, India), and the
University of Southampton (Southampton, United Kingdom).[5] The proposed experiment seems to have the capability of testing the quantumness of an object, regardless of its mass or energy.[6] The proposed experiment uses the quantum mechanical principle that measuring an object can change its quantum nature.[6] Instead of a single measurement made at successive times, the experiment is based on two different measurements to produce a mass-independent probing of quantumness in an
harmonic oscillator.[5]
The proposed experiment examines an harmonic oscillator at different times. A
beam of light examines the object during one half of its
area of
oscillation to roughly determine its
position as whether the object is in that half of its oscillation, or not. Another beam of light senses its position farther along in the harmonic cycle.[6] If the object is quantum, the first position measurement by the first light beam will collapse its wavefunction and change where it will be at the second measurement.[6] However, if the object is
classical, the first observation will have no affect.[6] This experiment can be done with current technologies using a
nanocrystal with trillions of
atoms as the object.[6] The
authors propose that the very massive (10
kilogram)
mirrors at the
Laser Interferometer Gravitational-Wave Observatory (LIGO), which
vibrate together as a single object, could be used in such an experiment.[6]
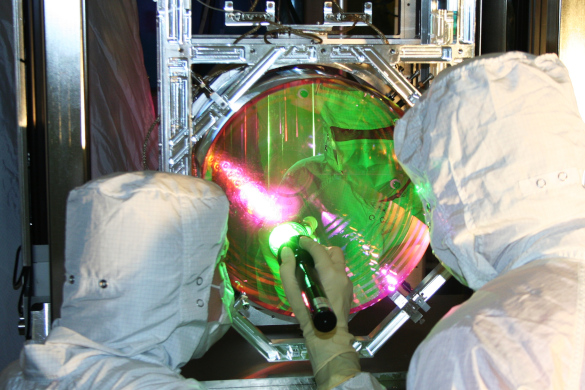
Technicians inspect the coating on one of LIGO's four mirrors. (LIGO Laboratory image, available here and here. Licensed under the Creative Commons CC BY License. Click for larger image.)
References:
- Certs Two Mints in One,
YouTube video by tvdays, January 21, 2022.
- WARNER LAMBERT COMPANY v. UNITED STATES, United States Court of Appeals,Federal Circuit, WARNER-LAMBERT COMPANY, Plaintiff-Appellant, v. UNITED STATES, Defendant-Appellee, No. 04-1489, Decided, May 11, 2005 (via findlaw.com).
- Louis Victor de Broglie, "Recherches sur sa Théorie Des Quanta (On the Theory of Quanta)," Ann. de Phys., Vol. 10, series III (January-February, 1925), A.F. Kracklauer, Trans., Foundation of Louis de Broglie, 2004.
- Louis de Broglie, "The Wave Nature of the Electron," Nobel Lecture, December 12, 1929.
- Debarshi Das, Dipankar Home, Hendrik Ulbricht, and Sougato Bose, "Mass-Independent Scheme to Test the Quantumness of a Massive Object," Phys. Rev. Lett., vol. 132, no. 3 (January 19, 2024), Article no. 030202, DOI:https://doi.org/10.1103/PhysRevLett.132.030202. This is an open source publication with a PDF file here.
- Experiment could test quantum nature of large masses for the first time, University College London Press Release, January 16, 2024.
Linked Keywords: Television; monochrome; mid-1960s; color television; technology; technologies; decade; backward compatibility; backwards-compatible; television set; television receiver; transmission (telecommunications); signal (electronics); reference; local oscillator; stabilize; stabilized; colorburst; signal pulse; interlaced video; image frame; television advertisement; television commercials; adolescence; teenage; twin; debate; debating; Certs; mint (candy); breath mint; candy; dispute resolution; resolved; announcer; United States Customs Service; tax; tariff; tariff class 2106.90.99; Warner-Lambert; Court of Appeals for the Federal Circuit; tariff class 3306.90.00; law; legally; YouTube video; nature; electron; matter; particle; electromagnetic radiation; wave; measurement; measure; matter wav; de Broglie hypothesis; hypothesize; France; French; physicist; Louis de Broglie (1892-1987); Hermann Minkowski (1864-1909); Max Abraham (1875-1922); vacuum wavelength; photon; Planck constant; momentum; equation; equate; energy; Albert Einstein; mass-energy equivalence; Planck relation; speed of light; velocity; science; Bachelor of Arts; history; mathematics; physics; Doctor of Philosophy; Ph.D.; thesis; Wikimedia Commons; Davisson-Germer experiment; experiment on electron diffraction; Clinton Davisson (1881-1958); Lester Germer (1896-1971); Bell Labs; Bragg's law; scatter; surface; crystal; nickel; electron diffraction; diffraction pattern; analogy; X-ray diffraction; electromagnetic radiation; electromagnetic wave; Nobel Prize in Physics; discovery of the wave nature of electrons; quantum nature; mass; environment (biophysical); quantum decoherence; collapse of quantum state; scientific literature; article; Physical Review letters; University College London (London, United Kingdom); Bose Institute (Kolkata, India); University of Southampton (Southampton, United Kingdom); harmonic oscillator; beam of light; area; oscillation; position (vector); classical physics; nanocrystal; atom; author; kilogram; mirror; Laser Interferometer Gravitational-Wave Observatory (LIGO); vibration; vibrate; technician; inspection; inspect; coating; LIGO Laboratory; Creative Commons CC BY License.