Radioactive Decay
August 30, 2010
One of the most curious things in physics is
radioactive decay. That some
isotopes of elements decay into other isotopes of the same element, or into isotopes of other elements, is well established. Everything is "explained" by the
half-life, the time it takes for half the number of nuclei of a particular isotope to decay into something else. Radioactive decay follows a nice
exponential law, but much is left unsaid. Why does a particular nucleus "decide" it should decay? Does it have an internal alarm clock that's set when it's first formed? If it did, it would be a very curious alarm clock, since the time is set so that on average we see the exponential decay we're accustomed seeing in an ensemble of the nuclei. The accepted explanation is that such decays require an
activation energy, and
quantum fluctuations of the vacuum push a nucleus over the top on occasion. Radioactive decay depends not just on the nucleus, but on its quantum environment. Like
Bertrand Russell, we need to look at a
lower turtle.
I wrote about some interesting experimental findings concerning radioactive decay in a
previous article (Radioactive Decay Surprise, September 04, 2008). A team from
Purdue University reported that the radioactive decay of
silicon-32 (
32Si) and
radium-226 (
226Ra) seemed to exhibit a small (0.1%) annual periodicity. The Purdue team did this analysis after they found that a massive solar flare on December 13, 2006, significantly decreased the decay of
manganese-54 (
54Mn) in their laboratory [1]. The decay rate of
54Mn dropped about a day and a half before the flare and continued as the flare progressed. The decay anomaly was noted both day and night, which was indicative that whatever was the cause traveled through the Earth without a problem. This would be true if the cause were solar neutrinos.
This isn't the first time that strange oscillations have been detected in radioactive decay and pinned on neutrinos. As I wrote in
another article (Nonexponential Decay, August 21, 2008), A group of physicists at the
Gesellschaft für Schwerionenforschung,
Darmalstadt,
Germany, discovered an oscillation in the radioactive decay of the
promethium isotopes,
140Pm and
142Pm. [4-7] These oscillations are very easy to see, since their amplitude is about 20% of the baseline exponential curve, and they have a period of about seven seconds. The Darmalstadt team conjectures that this is the effect of
neutrino oscillation. For those who like a good mystery, another team from Lawrence Berkeley National Laboratory tried a similar, though not exact, experiment, and they saw no oscillation.[8]
Among the possible mechanisms that the Purdue team proposed is that the
Sun may produce a field that changes the value of the
fine structure constant depending on distance. [2] This seems unlikely, since the fine structure constant agrees with theory to eleven decimal places. Some internet commentators think the result can be ascribed to experimental error caused by seasonal temperature variation of the measurement apparatus [1] Furthermore, the
Earth's distance from the Sun varies by a few percent, but the effect is seen only at the 0.1% level. The data, however, were quite convincing, as the figure for
radium-226 shows, and the Purdue team has published subsequent papers in several journals with the conclusion that experimental error is not to blame.[3]
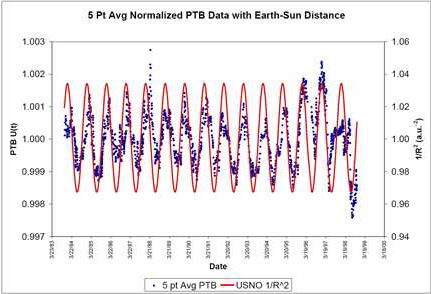
Deviation of 226Ra radioactive decay from an exponential law vs. 1/R2, where R is the Earth-Sun distance.
Peter Sturrock, an emeritus professor from
Stanford University, examined the data of the Purdue team and suggested that they look for a periodicity with the rotation rate of the Sun, also. They found that there was a 33 day periodicity, which falls into the range of the surface rotation rate of the sun, which varies from about 25 days at the equator to about 34 days at the poles.[9] Says Sturrock,[3]
"It's an effect that no one yet understands... Theorists are starting to say, 'What's going on?' But that's what the evidence points to. It's a challenge for the physicists and a challenge for the solar people too... (If not the neutrinos) It would have to be something we don't know about, an unknown particle that is also emitted by the sun and has this effect, and that would be even more remarkable.".
At this point, many groups are analyzing their archived data in attempts to verify the effect. An analysis of
90Sr-
90Y,
60Co and
239Pu decay by a group from the Institute for Time Nature Explorations of the
Lomonosov Moscow State University,
Moscow, Russia, did not see any solar flare effect.[10] The
Cassini spacecraft uses plutonium-238 decay as a source of energy in its
radioisotope thermoelectric generator, and the spacecraft's trajectory took it through much of the solar system from 0.6 astronomical units to 1.7 astronomical units (An
astronomical unit (AU) is equal to the mean distance between the Earth and the Sun). An analysis of the spacecraft's power output showed no correlation as a function of distance from the Sun.[11-12] At a 90% confidence level, the supposed variation with (1/R
2) is less than 0.84 x 10
-4, and with (1/R) is less than 0.99 x 10
-4.[12]
References:
- Do nuclear decay rates depend on our distance from the sun? (arXiv Blog, August 29, 2008).
- Jere H. Jenkins, Ephraim Fischbach, John B. Buncher, John T. Gruenwald, Dennis E. Krause and Joshua J. Mattes, "Evidence for Correlations Between Nuclear Decay Rates and Earth-Sun Distance" (arXiv Preprint, August 25, 2008).
- Dan Stober, "The strange case of solar flares and radioactive elements," Stanford Report, August 23, 2010.
- Bertram Schwarzschild, "Physics Update: Nonexponential Nuclear Decay," Physics Today, vol. 61, no. 8 (August 2008), p.24.
- Philip M. Walker, "Nuclear physics: A neutrino's wobble?", Nature, vol. 453, no. 7197 (June 12, 2008), pp. 864-865.
- Graph of oscillating decay data for 142Pm.
- Yu.A. Litvinova, et al.,"Observation of non-exponential orbital electron capture decays of hydrogen-like 140Pr and 142Pm ions," Physics Letters B, vol. 664, no. 3 (June 19, 2008), pp. 162-168.
- P.A. Vetter, R. M. Clark, J. Dvorak, S. J. Freedman, K. E. Gregorich, H.B. Jeppesen, D. Mittelberger, and M. Wiedeking, "Search for Oscillation of the Electron-Capture Decay Probability of 142Pm" (arXiv Preprint, July 3, 2008).
- The Sun page on Wikipedia.
- A.G. Parkhomov, "Effect of radioactivity decrease. Is there a link with solar flares?" (arXiv Preprint, May 15, 2010).
- Ian O'Neill, "Using Cassini to Test Radioactive Decay Rate Variation," Astroengine, September 26, 2008.
- Peter S. Cooper, "Searching for modifications to the exponential radioactive decay law with the Cassini spacecraft,"(arXiv Preprint, September 24, 2008).
- Andrew Moseman, "Scientist Smackdown: Are Solar Neutrinos Messing With Matter?," Discover Blog, August 26, 2010.
Permanent Link to this article
Linked Keywords: radioactive decay; isotopes; half-life; exponential law; activation energy; quantum fluctuations of the vacuum; Bertrand Russell; turtles; Purdue University; silicon-32; radium-226; manganese-54; Gesellschaft für Schwerionenforschung; Darmalstadt, Germany; promethium-140; promethium-142; neutrino oscillation; Sun; fine structure constant; Earth's distance from the Sun; Stanford University; Lomonosov Moscow State University; Moscow, Russia; Cassini spacecraft; radioisotope thermoelectric generator; astronomical unit.