Dye-Sensitized Solar Cells
June 23, 2010
Michael Grätzel, a.k.a., Michael Graetzel, has won the 2010
Millennium Technology Prize, a prize that's funded jointly by
Finland and industrial organizations [1]. Graetzel was awarded the prize for his development of the eponymous
Graetzel cell, the low-cost dye-sensitized solar cell. The value of the award is slightly more than a million dollars, a amount that puts it nearly on par with the better-known
Nobel Prizes. Graetzel has authored two books and more than 500 papers, and he's a listed inventor on more than forty patents [2].
Why has Finland funded this prize? Finland has become a hotbed of technology, with cellphone and communications equipment manufacturer
Nokia and instrument manufacturer
Vaisala being just two examples. I would be remiss if I didn't mention also
Linus Torvalds, originator of
Linux, who was born in
Helsinki, Finland, and graduated from the
University of Helsinki with a Master's degree in computer science.
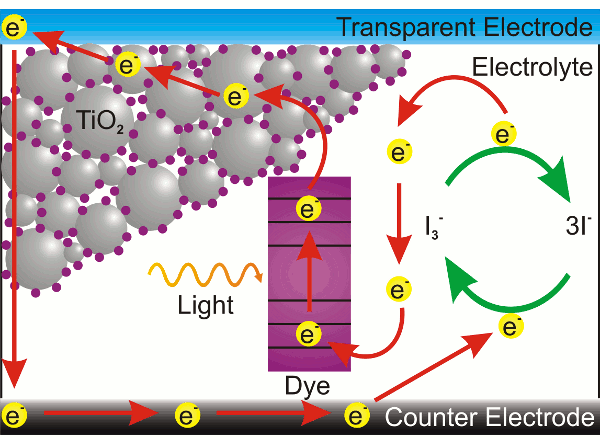
The Graetzel cell has lower efficiency than other solar cells, but its robustness and low cost make it an attractive renewable energy candidate. The architecture of a Graetzel cell is quite simple (see the figure). A glass plate is coated with a transparent electrode (typically
fluorine-doped
tin oxide, SnO
2:F), and the transparent electrode is coated with a thin, porous layer of
titanium dioxide (TiO
2). The TiO
2 layer absorbs light only in the ultraviolet, but it has a high surface area that's coated with a photosensitive dye,
ruthenium-polypyridine. This top plate assembly is then sealed with an
iodine electrolyte and a metal backside electrode. Unfortunately, the metal most compatible with the iodine electrolyte is
platinum, so there's a small cost issue here.
Dye Sensitized Solar Cell Scheme
A solar
photon entering the Graetzel cell will excite dye molecules on the TiO
2, and an electron will be injected into the TiO
2 conduction band and appear at the transparent
anode. The circuit is completed by the dye molecule capturing an electron from the iodine electrolyte, so there will be a current flow between the transparent electrode and the metal electrode.
This type of solar cell is so simple in construction, that Graetzel published a paper [3] describing a solar cell that can be made as an undergraduate laboratory exercise. In this case, the dye is a natural
anthocyanin dye extracted from berries. A complete experimental protocol using dye extracted from blackberries or raspberries can be found in Ref. 4
References:
- Tarmo Virki, "Solar-Cell Inventor Wins Millennium Prize," PCMag.com (June 9, 2010).
- Michael Grätzel Page at Wikipedia.
- Greg P. Smestad and Michael Gratzel, "Demonstrating Electron Transfer and Nanotechnology: A Natural Dye-Sensitized Nanocrystalline Energy Converter," J. Chem. Educ., vol. 75, no. 6 (1998), p. 752f..
- Paul Fuierer, "TEACHER GUIDE: Nanocrystalline Solar Cells," University of New Mexico (Undated PDF File).
Permanent Link to this article