Advanced Aluminum Alloys
November 6, 2017
In my
youth, there were many
television commercials for Reynolds Wrap
aluminum foil, the
cooking foil introduced in 1947 by
Reynolds Metals, the second-largest
aluminum company in the
United States after
Alcoa. Just as
photocopying is often referred to
generically as "
xeroxing," in those days, aluminum foil of any sort was often called "Reynolds Wrap." Reynolds was eventually acquired by Alcoa in the year 2000.
Aluminum is a
metal with ideal
properties for many applications. It's
abundant in nature,
lightweight, and it's
corrosion-resistant; however, until about 150 years ago, aluminum was rarely used, since it was difficult to
extract from its ores. While processes to
reduce iron oxide to pure
iron have existed since the
Iron Age, about 3,000 years ago,
aluminum oxide is much, much harder to reduce.
That's because aluminum oxide is a far more stable compound than iron oxide, as shown by the difference in their
free energies of formation (ΔG
f), as shown for two representative
temperatures in the following table.[1]
| Oxide |
ΔGf, 1000 K
kcal/mole |
ΔGf, 1500 K
kcal/mole |
| Fe2O3 |
-134.082 |
-104.585 |
| Al2O3 |
-325.397 |
-285.975 |
Aluminum was first
isolated in 1827, and early aluminum was prepared by a reduction of
gaseous anhydrous aluminum chloride by liquid
sodium metal at high temperature; viz,
Al2Cl6(g) + 6Na(l) -> 2Al(s) + 6NaCl(s)
In 1884, aluminum was as valuable as
silver, about a
dollar an
ounce ($25/ounce in
today's money), and the
Washington Monument was topped with an aluminum
apex in that year (see figure). This apex, intended as a
lightning rod, was a solid
pyramid of aluminum about a
foot high, weighing about 2.85 kg. The aluminum contained some
alloying elements, including iron (1.70-1.90%), and
silicon (0.55-0.61%), in the aluminum matrix (97.49-97.75%).[2]

Master Mechanic, P. H. McLaughlin, fitting the aluminum apex on the Washington Monument, 1884.
(Harper's Weekly illustration by S.H. Nealy, United States Library of Congress Prints and Photographs division digital ID cph.3b44599, via Wikimedia Commons.)
The "Aluminum Age" started in 1886 with the
invention of an
Electrochemical process for the extraction of aluminum from
molten aluminum fluoride. This process, now called the
Hall–Héroult process, was independently
invented by
American chemist,
Charles Martin Hall and
French inventor,
Paul Héroult. Hall founded the company that would become Alcoa in
Pittsburgh in 1888.
In the Hall–Héroult process,
cryolite (Na
3AlF
6) is melted by heated to about 1,000 °C (1,830 °F). To increase
energy efficiency,
aluminium fluoride (AlF
3) is added to form a reduced
melting point composition (see figure). Application of an
electric current through a
carbon cathode and
anode deposits aluminum as a
liquid at the cathode. The molten aluminum sinks to the bottom of the
electrochemical cell where it is collected.
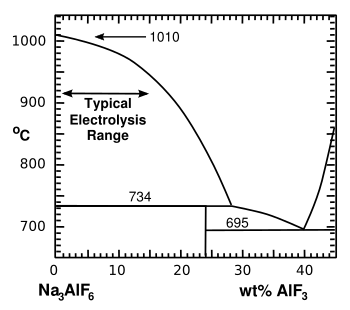
Cryolite-rich portion of the cryolite-aluminum fluoride phase diagram.
(Illustration by the author using Inkscape.)
(Click for larger image.)
A considerable quantity of
electric power is needed for this process. It takes about 25-30
kilowatt-hours of
electricity to produce two
kilograms of aluminum, which is about the daily electrical consumption of an average US home. That's one reason why
recycling of aluminum is important.
While the lightweight quality of aluminum is important to
aviation and
spacecraft applications,
engineers still yearn for even lower weight materials; thus, the quest for
structural graphene materials. That's why
chemists from
Utah State University and
Russia's Southern Federal University have used
computational chemistry to design a new
crystalline form of aluminum that is so light it will
float on water.[3-4]
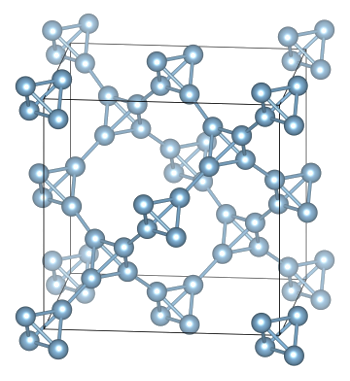
The new allotrope of aluminum has the diamond lattice structure in which all carbon atoms are replaced by aluminum tetrahedra.
(Image: Iliya Getmanskii, Southern Federal University, Russia, via Utah State University.
The
research team, whose work was supported by the
US National Science Foundation and the
Russian Ministry of Science and Education, used
density functional calculations to design this
metastable allotrope of aluminum that has yet to be
synthesized.[3-4] The
crystal structure is essentially a
diamond lattice in which the carbon
atom positions are now
tetrahedra of Al
4. This structure has a calculated
density of just 0.61
g/cm3, so it would float on water (density, 1.00 g/cm
3).[3] The density of conventional aluminum is 2.7 g/cm
3. Calculations indicate that this new form of aluminum is a
semimetal, and it should be a highly
plastic material.[3]
Says
Alexander Boldyrev, a
professor of
Chemistry and Biochemistry at Utah State University,
"An amazing aspect of this research is the approach: using a known structure to design a new material... My colleagues' approach to this challenge was very innovative... They started with a known crystal lattice, in this case, a diamond, and substituted every carbon atom with an aluminum tetrahedron... Of course, it's very early to speculate about how this material could be used. There are many unknowns. For one thing, we don’t know anything about its strength."[4]
While this new allotrope of aluminum has yet to be synthesized, researchers at
HRL Laboratories, Malibu, California, have developed a
3D method for printing high-strength
aluminum alloys, including types
Al7075 and
Al6061 that are useful for
aircraft and
automobile parts.[5-6] The HRL Laboratories method is also amenable to the
additive manufacture of many other useful alloys, such as
high-strength steels, and the
nickel-based
superalloys used in
turbine engines.[6]
Printing of metals typically proceeds by
laser heating of applied thin alloy
powder layers so they melt and
solidify, but this isn't possible for
unweldable high-strength aluminum alloys such as Al7075 or Al6061. These alloys exhibit
hot cracking in which the metal part can be pulled apart in layers.[6] Of the more than 5,000 common alloys, just a few like AlSi
10Mg,
TiAl
6V4,
CoCr and
Inconel 718, can be laser printed.[5] Melting and solidification of the others changes the alloy
microstructure to produce large
columnar grains and periodic cracks.[5]
The HRL Laboratory process overcomes these problems by addition of specially selected
nanoparticles to the unweldable alloy powders. As the laser melts the powder and solidification proceeds, these nanoparticles act as
nucleation sites to produce the desired alloy microstructure, thus eliminating hot cracking.[6] As a result, the printed alloy retains its full alloy strength.[6] The nanoparticles are uniformly distributed on the surface of the conventional powder grains to act as nucleation sites that control solidification.[5-6] For the aluminum alloys,
zirconium-based nanoparticles were used.[6]
A 3D-printed aluminum alloy component.
(Still image from an HRL Laboratory YouTube Video.)[7]
The resultant printed parts were crack-free, with an
equiaxed fine-grained microstructure; that is, the alloy grains were roughly equal in length, width and height. This produced material strengths that were comparable to that of
wrought material.[5] This technique can be applied, also, to non-weldable nickel superalloys and
intermetallics, as well as in joining,
casting and
injection molding operations in which solidification cracking is a common issue.[5] Says Hunter Martin, an engineer at
HRL's Sensors and Materials Laboratory,
co-principal investigator of the research project, and a
Ph.D. candidate at the
University of California, Santa Barbara,
"We're using a 70-year-old nucleation theory to solve a 100-year-old problem with a 21st century machine... Our first goal was figuring out how to eliminate the hot cracking altogether. We sought to control microstructure and the solution should be something that naturally happens with the way this material solidifies."[6]
References:
- L. B. Pankratz, "Thermodynamic Properties of Elements and Oxides," U. S. Bureau of Mines Bulletin 672, U. S. Government Printing Office (1982).
- George J. Binczewski, "The Point of a Monument: A History of the Aluminum Cap of the Washington Monument," JOM, vol. 47, no. 11 (1995), pp. 20-25.
- Iliya V. Getmanskii, Vitaliy V. Koval, Ruslan M. Minyaev, Alexander I. Boldyrev, and Vladimir Isaak Minkin, "Supertetrahedral Aluminum - a New Allotropic Ultra-Light Crystalline Form of Aluminum," J. Phys. Chem. C, Just Accepted Manuscript (September 18, 2017), DOI: 10.1021/acs.jpcc.7b07565.
- Ultra-Light Aluminum: USU Chemist Reports Material Design Breakthrough, Utah State Today, Friday, September 22, 2017.
- John H. Martin, Brennan D. Yahata, Jacob M. Hundley, Justin A. Mayer, Tobias A. Schaedler, and Tresa M. Pollock, "3D printing of high-strength aluminium alloys," Nature, vol. 549, no. 7672 (September 21, 2017), pp. 365-369, doi:10.1038/nature23894.
- Metallurgy Breakthrough: HRL Engineers 3D Print High-Strength Aluminum, Solve Ages-Old Welding Problem Using Nanoparticles, HRL Laboratories Press Release, September 20, 2017.
- Metallurgy Breakthrough: 3D Printing High-Strength Aluminum, HRL Laboratories YouTube Video, September 20, 2017.
Linked Keywords: Youth; television commercial; aluminum foil; cooking; Reynolds Group Holdings; Reynolds Metals; aluminum; United States; Alcoa; photocopier; photocopying; generic trademark; xeroxing; metal; physical property; abundances of the elements; nature; lightweight; corrosion-resistant; mining; extract from ore; melting; reduce; iron oxide; iron; Iron Age; aluminum oxide; standard Gibbs free energy of formation; emperature; calorie; kcal; mole; Fe2O3; Al2O3; isolate; gas; gaseous; anhydrous; aluminum chloride; sodium metal; silver; dollar; ounce; time value of money; today's money; Washington Monument; apex; lightning rod; pyramid; foot; alloy; alloying element; silicon; Harper's Weekly; United States Library of Congress; Wikimedia Commons; invention; electrochemistry; electrochemical; molten; aluminum fluoride; Hall–Héroult process; invention; invented; American; chemist; Charles Martin Hall; French; Paul Héroult; Pittsburgh; cryolite; energy efficiency; melting point; electric current; carbon; cathode; anode; liquid; electrochemical cell; cryolite-aluminum fluoride phase diagram; Inkscape; electric power; kilowatt-hour; electricity; kilogram; recycling; aviation; spacecraft; engineer; structural; graphene; Utah State University; Russia; Southern Federal University; computational chemistry; crystal; crystalline; buoyancy; float on water; allotropy; allotrope; diamond cubic; diamond lattice structure; atom; tetrahedral symmetry; tetrahedron; research; US National Science Foundation; Russian Ministry of Science and Education; density functional theory; density functional calculation; metastability; metastable; chemical synthesis; synthesize; density; kilogram per cubic meter; g/cm3; semimetal; plasticity; plastic; Alexander Boldyrev; professor; collaboration; colleague; innovation; innovative; speculative reason; speculate; strength of materials; HRL Laboratories, Malibu, California; 3D printing; aluminum alloy; Al7075; Al6061; aircraft; automobile; additive manufacture; high-strength steel; nickel; superalloy; gas turbine engine; laser; powder; freezing; solidify; welding; unweldable; hot cracking; magnesium; Mg; titanium; Ti; vanadium; V; cobalt; Co; chromium; Cr; Inconel 718; microstructure; columnar grain; nanoparticle; nucleation; zirconium; YouTube Video; equiaxed crystals; fine-grained; metalworking; wrought; intermetallic; casting; injection molding; HRL Sensors and Materials Laboratory; principal investigator; Ph.D. candidate; University of California, Santa Barbara; 21st century.