Nanocellulose Capacitors
January 14, 2016
My
father was a
carpenter, so our
material of choice was always
wood. There were bits and pieces of wood, such as
wall studs and
plywood, in various neat piles in the
garage and the
cellar workshop. These were a great source of materials for my many
science fair projects. As they say,
if all you have is a hammer, everything looks like a nail, and my father even repaired a broken
automobile seat with a piece of wood.
Wood is a great
renewable resource, and it's no wonder that most
houses are
framed with wood. For the past
century or more, the typical home is
constructed using
balloon framing. This type of construction, in which many smaller wall studs replaced fewer and heavier supporting posts at corners and principal junctions, made for easier house framing. The older construction practice required skilled
joining, while the balloon houses could just be
nailed together.
Wood is a combination of several
chemical compounds, but its major constituent (about 42%) is
cellulose. Cellulose is a
crystalline polymer that derives considerable
strength from the
hydrogen bonding between straight, parallel chains of the cellulose polymer (see figure). I wrote about cellulose in an
earlier article (Cellulosic Carbon, April 30, 2014).
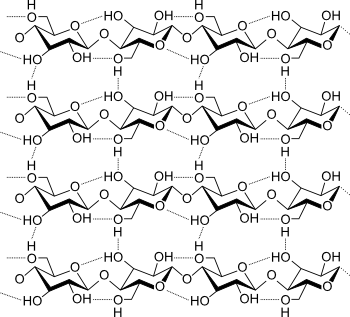
Polymer chains of cellulose.
The polymer chains are straight, and the strength of wood derives from hydrogen bonding, not the coiling or branching found in other polymers.
(Illustration by Luca Laghi, via Wikimedia Commons.)
Paper is a principal wood product; and, as you affirm every time you buy another
ream of paper for your
printer, or haul another load of
junk mail to the
recycle center, paper is inexpensive. That's why a team of
scientists from
Linköping University (Norrköping, Sweden), the
Technical University of Denmark (Roskilde, Denmark), the
University of Kentucky (Lexington, Kentucky), and the
KTH Royal Institute of Technology (Stockholm, Sweden) has looked at paper as an
energy storage medium. They processed sheets of
nanocellulose to form
capacitors with a large energy density.[1-3]
A quick look at the
equations describing the simplest capacitor, the parallel plate capacitor, gives us an idea of what needs to be improved to give higher energy storage ability.
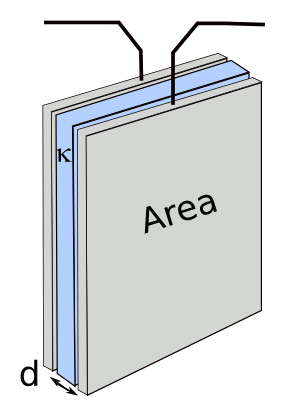 |
C = κεoA/d
Q = C V
I = ∂Q/∂t
W = (1/2) C V2
V = Vo e(-t/RC)
Q = C Vo e(-t/RC)
I = (Vo/R) e(-t/RC)
where C is capacitance, κ is the dielectric constant, εo is the permittivity of free space, A is the area of the capacitor plates, d is the plate separation, Q is the charge, V is the voltage, Vo is the initial voltage, I is the current, W is the stored energy, t is time, and R is the load resistance.
|
A convenient unit for
εo, the permittivity of free space, is 8.854 x 10
-12 farads per meter . If you use farads as the units of capacitance, the stored energy W will have units of joules. A watt is a joule per second. As we can see from these equations, a large plate area, small plate separation and high dielectric constant lead to high capacitance; and, thereby, high energy storage at high voltage.
The research team, led by scientists at the Laboratory of Organic Electronics of Linköping University, has developed what they call "power paper," a composite of nanocellulose and the
conductive polymer,
PEDOT:PSS (poly(3,4-ethylene-dioxythiophene):poly(styrene-sulfonate).[1-3] The energy storage principal is capacitance, and
supercapacitors are similarly used for energy storage applications. Power paper is different, since it has a three-dimensional structure that's easy to produce in thick sheets.[3] A fifteen
centimeter disk of the material, a few
millimeters thick, has a capacitance of up to one farad, about the same capacitance of supercapacitors.

This flexible piece of power paper has a one farad capacitance.
(Linköping University image by Thor Balkhed.)
One other distinguishing characteristics of the material is its ability to be
recharged hundreds of times, and each charge only takes a few seconds.[3] A piece of power paper has the highest charge (1
coulomb) and highest capacitance (2 farads) in an
organic electronic device.[1,3] The material also has the highest measured
current (1
ampere) in an organic conductor, and the highest
transconductance (1
siemen) when made into a
transistor.[1,3]
The nanocellulose is created using
high pressure water, which breaks cellulose fibers into strands as thin as 20
nanometer in diameter.[3] The PEDOT:PSS conducting polymer is added while the nanocellulose is still in a
water solution, and the polymer forms a thin coating around the fibers.[3] Says Linköping University
doctoral student,
Jesper Edberg, "The covered fibers are in tangles, where the liquid in the spaces between them functions as an
electrolyte."[3]
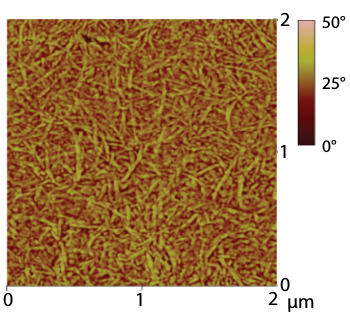
A phase atomic force microscopy image of a 70 micrometer thick film of vacuum-dried power paper
(Fig. 3a of ref. 1, licensed under a Creative Commons Attribution License.)
Because of its simultaneous high
ionic conductivity and
electronic conductivity, power paper has an exceptional capacity for energy storage.[3] It has the further advantages that it's made from simple materials, cellulose and an inexpensive polymer. It's lightweight, contains no
toxic heavy metals or other
toxic chemicals, and it's
waterproof.[3] The research team is looking into an
industrial scale process for power paper production.[3] This research was
funded by the
Knut and Alice Wallenberg Foundation, and the
Swedish Foundation for Strategic Research.[3]
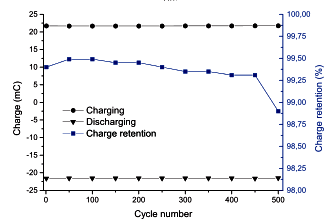
Charge and discharge stability of a power paper capacitor.
(Fig. 5d of ref. 1, licensed under a Creative Commons Attribution License.)
![]()
References:
- Abdellah Malti, Jesper Edberg, Hjalmar Granberg, Zia Ullah Khan, Jens W. Andreasen, Xianjie Liu, Dan Zhao, Hao Zhang, Yulong Yao, Joseph W. Brill, Isak Engquist, Mats Fahlman, Lars Wågberg, Xavier Crispin, and Magnus Berggren, "An Organic Mixed Ion–Electron Conductor for Power Electronics," Advanced Science (December 2, 2015), doi: 10.1002/advs.201500305. This is an open access publication with a PDF file here.
- Supplementary Information for ref 1.
- Storing electricity in paper - An organic mixed ion-electron conductor for power electronics, Linköping University Press Release, December 3, 2015. The press release is also available here.
Permanent Link to this article
Linked Keywords: Father; carpentry; carpenter; material; wood; wall stud; plywood; garage; basement; cellar; workshop; science fair; law of the instrument; if all you have is a hammer, everything looks like a nail; automobile; seat; renewable resource; house; balloon framing; century; construction; woodworking joint; joining; nail; chemical compound; cellulose; crystal; crystalline; polymer; ultimate tensile strength; hydrogen bond; hydrogen bonding; cellulose; branching; Luca Laghi; Wikimedia Commons; paper; ream; printer; junk mail; recycling; recycle center; scientist; Linköping University (Norrköping, Sweden); Technical University of Denmark (Roskilde, Denmark); University of Kentucky (Lexington, Kentucky); KTH Royal Institute of Technology (Stockholm, Sweden); energy storage; nanocellulose; capacitor; energy density; equation; parallel plate capacitor; capacitance; relative permittivity; dielectric constant; vacuum permittivity; permittivity of free space; area; electric charge; voltage; electric current; energy; time; resistance; farad; meter; joule; watt; composite material; conductive polymer; PEDOT:PSS (poly(3,4-ethylene-dioxythiophene):poly(styrene-sulfonate); electric double-layer capacitor; supercapacitor; centimeter; millimeter; Thor Balkhed; rechargeable battery; recharge; coulomb; organic electronic; ampere; transconductance; siemen; transistor; high pressure; water; nanometer; aqueous solution; postgraduate education; doctoral student; Jesper Edberg; electrolyte; atomic force microscopy; micrometer; vcuum drying; vacuum-dried; Creative Commons Attribution License; ionic conductivity; electronic conductivity; toxic heavy metal; toxic chemical; wet strength; industrial scale; funding; Knut and Alice Wallenberg Foundation; Swedish Foundation for Strategic Research; charge cycle; chemical stability.