Honeycombs and Herringbones
May 31, 2013
Objects sometimes have hidden
properties that change their operating characteristics. Unintentionally
magnetized tools, for example, are the bane of many a
technician. They upset your arrangement of parts by their magnetism, and that's why some tools are fabricated from
non-magnetic steels. Other tools purposely use magnetism to aid in their function, such as the magnetized
screwdrivers that help hold a
bolt in place while you're aligning it to a
threaded hole.
I have a
soldering iron whose tip is sometimes annoyingly magnetic, moving some types of
components as I try to solder their electrical leads. The interesting thing is that the magnetism of this soldering iron is a side-effect of its operating principle.
Carl E. Weller was issued US
Patent No. 2,951,927 on September 6, 1960, for an "Electric Soldering Iron" with a novel
temperature regulating mechanism. When the soldering iron tip got too hot, an inner element would lose its magnetism and
thermally disconnect the tip from the electrical heat source.[1]

Magnetic attraction of a Weller soldering iron tip.
Carl Weller's patented design for keeping a soldering iron tip at the proper temperature has a magnetic slug acting as a thermal switch. Heat decreases the magnetization of a ferromagnetic material, eventually extinguishing it at the Curie temperature.
(Photo by the author)
A recent article in
Nature Chemistry illuminates an unexpected hidden property of a
nanomaterial.[2-3] This work was the joint research of a huge team from three US
universities, the
University of Pennsylvania (Philadelphia, Pennsylvania), the
University of Michigan (Ann Arbor, Michigan) and the
Massachusetts Institute of Technology (Cambridge, Massachusetts); and one company,
Intelligent Material Solutions, Inc. (Princeton, New Jersey).[2]
As is typical in the
sciences, this work started with an
unexpected discovery, this one by
Christopher Murray, a
professor of
chemistry at the University of Pennsylvania, involving rafted assemblages of
nanoscale crystals of
lanthanide fluorides. These crystals were prepared from solution, and their size was limited to about 100
nanometers by the addition of
oleic acid, which stuck to the sides of the crystallites and prevented additional growth.
The
lanthanide contraction is the name given to the
phenomenon that the
ionic radius of the lanthanide elements shrinks as the
atomic number increases, while their
chemical properties remain roughly the same. By using different lanthanide elements in their crystals, the Penn scientists were able to modify the
crystal bonding and prepare crystallites of different shapes.
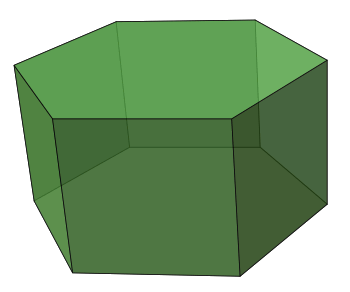
The crystal habit of the early lanthanide fluorides is the hexagonal prism. This changes to a rhombic prism for the later elements.
(Image by R. A. Nonenmacher, via Wikimedia Commons.)
The crystal habit of the early lanthanide fluorides is the
hexagonal prism, while the later lanthanides form
rhombic prism crystals. By changing the
elements, the Penn team was able to vary the prism shape. These nanocrystals were aggregated into close-packed
two-dimensional arrays by spreading a mixture of them in
hexane onto a layer of
ethylene glycol.[2-3] As the
solvent evaporated, the crystals were pulled together into the dense packing. The
diamond-like shapes and extended
hexagons packed as expected. The hexagons arrayed themselves as a stretched
honeycomb, which is as would have been expected.
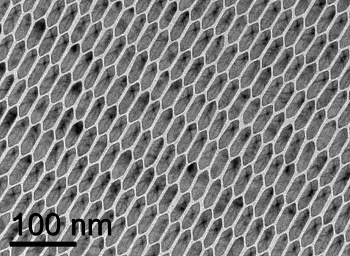
Nanoscale honeycomb pattern of hexagonal crystallites. In this case, elongated hexagonal prisms array themselves into the expected honeycomb pattern.
(Photomicrograph by Xingchen Ye of the University of Pennsylvania, via University of Michigan.)
When the hexagons were nearly perfect, with all sides nearly the same length, the scientists were surprised to find that the organization was a more complicated, alternating
herringbone pattern, as shown in the figure. At that point, the Penn team sought the advice of a University of Michigan team led by
Sharon Glotzer, a professor of
materials science and engineering.[4] The Michigan team has experience in
computer modeling of how shapes assemble.[3] I wrote about Glotzer's research in two previous articles (
Entropic Order, August 20, 2012 and
Packing and Filling, May 17, 2012)
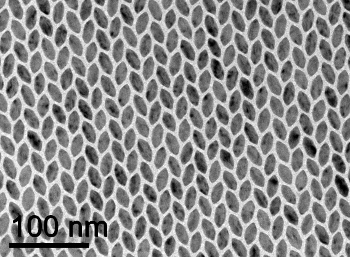
Nanoscale herringbone pattern of hexagonal crystallites.
(Photomicrograph by Xingchen Ye of the University of Pennsylvania, via the University of Michigan.)
The initial modeling showed that if the fundamental interaction between the crystallites was by their shape, only, the elongated hexagons should form the honeycomb pattern, and not the herringbone.[3] This result indicated that
interfacial forces were in play, and they found that if the edges forming the points were stickier than the remaining two sides, the herringbone pattern would result.[3]
Further modeling at MIT showed that the coating at the crystallite surface caused the differential stickiness. Since the arrangement of fluorine
atoms on the crystallite
facets differs, more of the
hydrocarbon will attach to the facets forming the points than the other facets.[3]
The result of the study is a method to regulate the arrangement of such nanoscale objects beyond their shape. Says Penn's Christopher Murray,
"The excitement in this is not in the herringbone pattern, it's about the coupling of experiment and modeling, and how that approach lets us take on a very hard problem."[3]
References:
- Carl E. Weller, "Electric Soldering Iron," US Patent No. 2,951,927, September 6, 1960.
- Xingchen Ye, Jun Chen, Michael Engel, Jaime A. Millan, Wenbin Li, Liang Qi, Guozhong Xing, Joshua E. Collins, Cherie R. Kagan, Ju Li, Sharon C. Glotzer and Christopher B. Murray, "Competition of shape and interaction patchiness for self-assembling nanoplates," Nature Chemistry, Published online, May 12, 2013, doi:10.1038/nchem.1651.
- Nano-breakthrough: Solving the case of the herringbone crystal, Joint University of Michigan and University of Pennsylvania Press Release, May 12, 2013.
- Sharon Glotzer's Web Site.
Permanent Link to this article
Linked Keywords: Material property; magnetization; magnetized; technician; austenite; non-magnetic steel; screwdriver; bolt; thread; soldering iron; electronic component; patent; temperature; regulator; automatic control mechanism; thermal conduction; thermal switch; ferromagnetism; ferromagnetic material; Curie temperature; Nature Chemistry; nanomaterial; university; University of Pennsylvania (Philadelphia, Pennsylvania); University of Michigan (Ann Arbor, Michigan); Massachusetts Institute of Technology (Cambridge, Massachusetts); Intelligent Material Solutions, Inc. (Princeton, New Jersey); science; serendipity; unexpected discovery; Christopher Murray; professor; chemistry; nanoscopic scale; nanoscale; crystal; lanthanide; fluoride; nanometer; oleic acid; organic compound; organic liquid; lanthanide contraction; phenomenon; ionic radius; atomic number; chemical property; crystal bonding; crystal habit; hexagonal prism; rhombic prism; element; Wikimedia Commons; chemical element; two-dimensions; hexane; ethylene glycol; solvent; evaporation; rhombus; diamond-like shape; hexagon; honeycomb lattice; honeycomb; herringbone pattern; Sharon Glotzer; materials science and engineering; computer modeling; adhesion; interfacial force; atom; facets; hydrocarbon; Carl E. Weller, "Electric Soldering Iron," US Patent No. 2,951,927, September 6, 1960.