
Nature Abhors Quasicrystals
January 16, 2012 The idiom that "Nature abhors a vacuum" is attributed to Baruch Spinoza, who developed a lot of interesting ideas that were certainly well ahead of his seventeenth century milieu. Scientists have the vacuum under control, both experimentally and theoretically, but what nature really abhors is crystals with five-fold symmetry. The reason for this is that space can't be filled with dodecahedrons, which are regular polyhedra with pentagonal sides. Johannes Kepler, in his book, Harmonices Mundi, showed that even when you drop down a dimension, pentagons are anathema. You can't fill a plane with regular pentagons, although irregular pentagons will do the job. The dodecahedron is one of the five Platonic solids, which are those solids that can be formed using the same regular polygon as faces. The others are the tetrahedron, cube, octahedron and icosahedron. Aristotle, in his book, On the Heavens, stated that the tetrahedron can fill space, but he was wrong. Platonic solid sculptures in Steinfurt, Germany. (Photo by Zumthie, via Wikimedia Commons).
The only Platonic solid that can fill space is the cube. If we relax our polyhedron requirement to solids that are formed through combinations of any regular polyhedra, not just one type, we get the Archimedean solids. Four objects in this class join the cube as space filling polyhedra. These are the triangular prism, hexagonal prism, truncated octahedron and gyrobifastigium.[1] Gyrobifastigium? I thought that mathematicians only drank coffee, but that's more like a beer pong word.
When atoms arrange themselves in crystals, they necessarily fill space, and they do this in the simplest way possible by ordering themselves into small polyhedral cells that repeat. Not surprisingly, the majority of crystal cells are cubic and hexagonal crystals. Looking at the first series of transition metals, scandium through zinc, we have four hexagonal crystals, four body-centered cubic crystals, and two face-centered cubic crystals.
As I summarized in a previous article (The 2011 Nobel Prize in Chemistry, October 7, 2011), no one believed Dan Shechtman in 1982 when he found five-fold symmetry in an alloy of aluminum that contained fourteen atomic percent manganese.[2-3] The material was novel, since it was formed by rapid solidification, a technique that inhibits motion of atoms as the material solidifies.
Shechtman's electron diffraction data indicated an icosahedral point group symmetry. Icosahedra can't fill space. Not surprisingly, it took two years for Shechtman to get his paper accepted for publication; but after Shechtman's publication, other scientists came forward with examples of the same thing.
In 1992 the International Union of Crystallography changed its definition of a crystal from "a regularly ordered, repeating three-dimensional pattern" to a solid with a "discrete diffraction diagram."[4] Shechtman was vindicated in his award of the 2011 Nobel Prize in Chemistry for his discovery of these quasicrystals, crystals that are ordered, but not periodic.
Platonic solid sculptures in Steinfurt, Germany. (Photo by Zumthie, via Wikimedia Commons).
The only Platonic solid that can fill space is the cube. If we relax our polyhedron requirement to solids that are formed through combinations of any regular polyhedra, not just one type, we get the Archimedean solids. Four objects in this class join the cube as space filling polyhedra. These are the triangular prism, hexagonal prism, truncated octahedron and gyrobifastigium.[1] Gyrobifastigium? I thought that mathematicians only drank coffee, but that's more like a beer pong word.
When atoms arrange themselves in crystals, they necessarily fill space, and they do this in the simplest way possible by ordering themselves into small polyhedral cells that repeat. Not surprisingly, the majority of crystal cells are cubic and hexagonal crystals. Looking at the first series of transition metals, scandium through zinc, we have four hexagonal crystals, four body-centered cubic crystals, and two face-centered cubic crystals.
As I summarized in a previous article (The 2011 Nobel Prize in Chemistry, October 7, 2011), no one believed Dan Shechtman in 1982 when he found five-fold symmetry in an alloy of aluminum that contained fourteen atomic percent manganese.[2-3] The material was novel, since it was formed by rapid solidification, a technique that inhibits motion of atoms as the material solidifies.
Shechtman's electron diffraction data indicated an icosahedral point group symmetry. Icosahedra can't fill space. Not surprisingly, it took two years for Shechtman to get his paper accepted for publication; but after Shechtman's publication, other scientists came forward with examples of the same thing.
In 1992 the International Union of Crystallography changed its definition of a crystal from "a regularly ordered, repeating three-dimensional pattern" to a solid with a "discrete diffraction diagram."[4] Shechtman was vindicated in his award of the 2011 Nobel Prize in Chemistry for his discovery of these quasicrystals, crystals that are ordered, but not periodic.
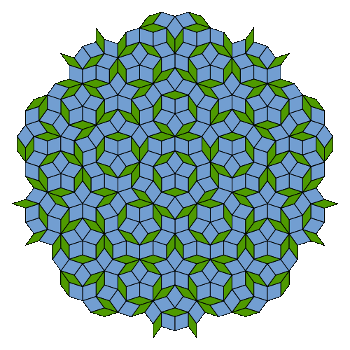 | A rather pretty tiling with five-fold rotational symmetry. This is a Penrose tiling, named after the mathematicial physicist, Roger Penrose. Penrose was issued a patent on his tiling in 1979.[5] (Via Wikimedia Commons). |
"...Our evidence indicates that quasicrystals can form naturally under astrophysical conditions and remain stable over cosmic timescales, giving unique insights on their existence in nature and stability."[7]
References:
- Space-Filling Polyhedron on Mathworld.
- D. Shechtman, I. Blech, D. Gratias and J.W. Cahn, "Metallic phase with long range orientational order and no translation symmetry,", Physical Review Letters, vol. 53, no. 20 (November 12, 1984), pp. 1951-1953.
- D. Levine and R. Steinhardt, "Quasicrystals: a new class of ordered structures," Physical Review Letters, vol. 53, no. 26 (December 24, 1984), pp. 2477-2480.
- Kenneth Chan, "Israeli Scientist Wins Nobel Prize for Chemistry," The New York Times, October 5, 2011.
- Roger Penrose, "Set of tiles for covering a surface," US Patent No. 4,133,152, January 9, 1979.
- Luca Bindi, Paul J. Steinhardt, Nan Yao and Peter J. Lu, "Natural Quasicrystals," Science, vol. 324, no. 5932 (June 5, 2009), pp. 1306-1309.
- Luca Bindi, John M. Eiler, Yunbin Guan, Lincoln S. Hollister, Glenn MacPherson Paul J. Steinhardt and Nan Yao, "Evidence for the extraterrestrial origin of a natural quasicrystal," Proc. Natl. Acad. Sci., doi: 10.1073/pnas.1111115109, January 3, 2012.
- Impossible crystals are 'from space', BBC News January 3, 2012.
- Richard Van Noorden, "The quasicrystal from outer space," Nature News, January 3, 2012.
- D. Shechtman, I. Blech, D. Gratias and J.W. Cahn, "Metallic phase with long range orientational order and no translation symmetry,", Physical Review Letters, vol. 53, no. 20 (November 12, 1984), pp. 1951-1953.