
Negative Thermal Expansion
November 11, 2011 The vast majority of materials expand when heated, an effect known as thermal expansion. Thermal expansion causes major problems in the manufacture of useful articles. Unless thermal expansion is taken into account during design, camera lenses will defocus, and parts carefully fit at room temperature will fracture as the temperature is increased. The linear thermal expansion of materials is quantified by the thermal expansion coefficient, which generally goes by the symbol, alpha (α),α = (1/L)(∂L/∂T)for which L is the gauge length, T is the temperature, and the dimensions are usually (1/°C). The following table lists the thermal expansion coefficient at room temperature (20°C) for a variety of materials.
| Material | α (10-6/°C) | Material | α (10-6/°C) |
| Ethanol | 250 | Carbon steel | 10.8 |
| Water | 69 | Glass | 8.5 |
| Mercury | 61 | Alumina | 7.8 |
| Aluminium | 23 | Tungsten | 4.5 |
| Stainless steel | 17.3 | Borosilicate glass | 3.3 |
| Copper | 17 | Invar | 1.2 |
| Nickel | 13 | Diamond | 1 |
| Concrete | 12 | Fused quartz | 0.59 |
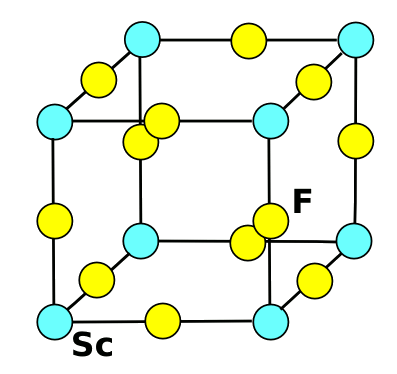 | The crystal structure of scandium fluoride. Drawing by author, rendered with Inkscape. |
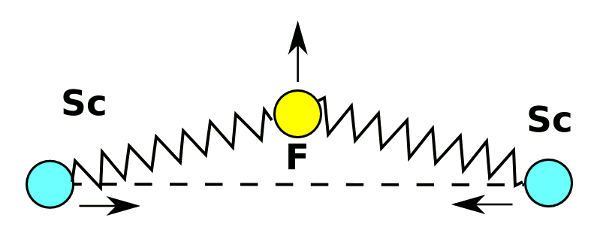 Principle of negative thermal expansion in scandium fluoride. The fluorine atoms vibrate in a transverse direction to the scandium atoms to pull them together. Drawing by author, rendered with Inkscape.
One curious finding is that the restoring force of the fluorine vibration is a function of the fourth power of the displacement. This quartic oscillation is unlike the quadratic (second power) oscillation that's found in atomic vibrations and harmonic oscillators.[7] Says Brent Fultz, study coauthor and Professor of Materials Science and Applied Physics at Caltech, "A nearly pure quantum quartic oscillator has never been seen in atom vibrations in crystals."[7]
The research team speculates that quartic oscillator materials may also be good thermal insulating materials. NTE materials can be combined with other materials to produce zero expansion materials, at least over a small temperature range. Such materials would be useful in optics, but also things as mundane as dental restoration.[6]
Principle of negative thermal expansion in scandium fluoride. The fluorine atoms vibrate in a transverse direction to the scandium atoms to pull them together. Drawing by author, rendered with Inkscape.
One curious finding is that the restoring force of the fluorine vibration is a function of the fourth power of the displacement. This quartic oscillation is unlike the quadratic (second power) oscillation that's found in atomic vibrations and harmonic oscillators.[7] Says Brent Fultz, study coauthor and Professor of Materials Science and Applied Physics at Caltech, "A nearly pure quantum quartic oscillator has never been seen in atom vibrations in crystals."[7]
The research team speculates that quartic oscillator materials may also be good thermal insulating materials. NTE materials can be combined with other materials to produce zero expansion materials, at least over a small temperature range. Such materials would be useful in optics, but also things as mundane as dental restoration.[6]
References:
- Martin Chaplin, Water Structure and Science - Explanation of the Density Anomalies of Water.
- Jason N. Hancock, Chandra Turpen, Zack Schlesinger, Glen R. Kowach and Arthur P. Ramirez, "Unusual Low-Energy Phonon Dynamics in the Negative Thermal Expansion Compound ZrW2O8," Phys. Rev. Lett., vol. 93, no. 22 (November 22, 2004), Document No. 225501
- David Lindley, "Bake, Shake, and Shrink," Physical Review Focus, vol. 14, no. 21 (November 22, 2004).
- Benjamin K. Greve, Kenneth L. Martin, Peter L. Lee, Peter J. Chupas, Karena W. Chapman and Angus P. Wilkinson, "Pronounced Negative Thermal Expansion from a Simple Structure: Cubic ScF3," J. Am. Chem. Soc., vol. 132, no. 44 (November 10, 2010), pp 15496-15498
- Chen W. Li, Xiaoli Tang, J. A. Muñoz, J. B. Keith, S. J. Tracy, D. L. Abernathy, and B. Fultz, "Structural Relationship between Negative Thermal Expansion and Quartic Anharmonicity of Cubic ScF3," Phys. Rev. Lett., vol. 107, no. 19 (November 4, 2011), Document No. 195504.
- Michael Schirber, "New Vibration in Material That Shrinks When Heated," Physics (APS), vol. 4, no. 90 (November 4, 2011).
- Marcus Woo, "An Incredible Shrinking Material," California Institute of Technology Press Release, November 4, 2011.
- Cal Tech, "An Incredible Shrinking Material," YouTube Video, November 4, 2011.
- Jason N. Hancock, Chandra Turpen, Zack Schlesinger, Glen R. Kowach and Arthur P. Ramirez, "Unusual Low-Energy Phonon Dynamics in the Negative Thermal Expansion Compound ZrW2O8," Phys. Rev. Lett., vol. 93, no. 22 (November 22, 2004), Document No. 225501